D-PLEX100 – PHASE 2 - SIGNIFICANT DECREASE IN THE RATE OF
SSI IN ABDOMINAL SURGERIES
![]()
- Design: accessor and patient blinded and double-arm randomized study
- Objective: safety and efficacy of D-PLEX100 + SoC (n=101) vs. SoC alone (n=100) in prevention of incision infection post-colorectal surgery
- Primary efficacy end-point: the decrease of infection rate as measured by the proportion of subjects with at least one abdominal incisional infection event, as determined by a blinded and independent adjudication committee, within 30 days post abdominal surgery.
![]()
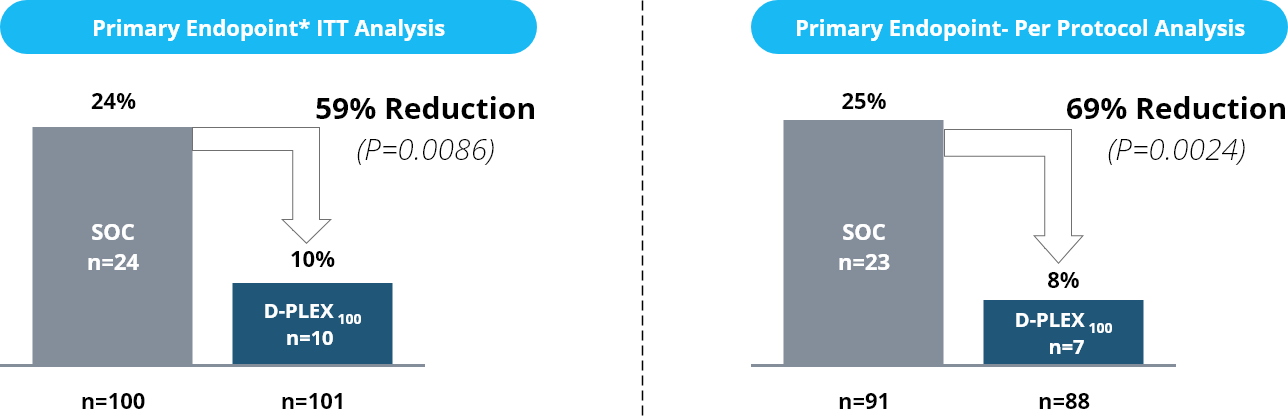
Primary Endpoint - infections & mortality – 30 days
5 deaths in the SoC treatment arm, as compared to zero in the D-PLEX100 treatment arm
within the first 60 days post-surgery (p=0.029). 3 deaths observed during the first 30 days
* PEP is the Combined SSI and mortality rate which is measured by the number and proportion of subjects with either an SSI event (as determined by the abdominal surgery) or mortality or any reason within 30 days post index surgery.
Note: The current standard of care for preventing SSIs involves the implementation of a range of treatment and prevention measures before, during and after surgery, including prophylactic antibiotic administration, antiseptic measures and wound care.
D-PLEX100 – PHASE 2 - ENCOURAGING EFFECT ON OVERALL
STERNAL WOUND HEALING
- Design: single-blinded and double-arm randomized study
- Objective: safety and efficacy of D-PLEX100 + SoC (n=60) vs. SoC alone (n=21) in prevention of sternal infection post-cardiac surgery
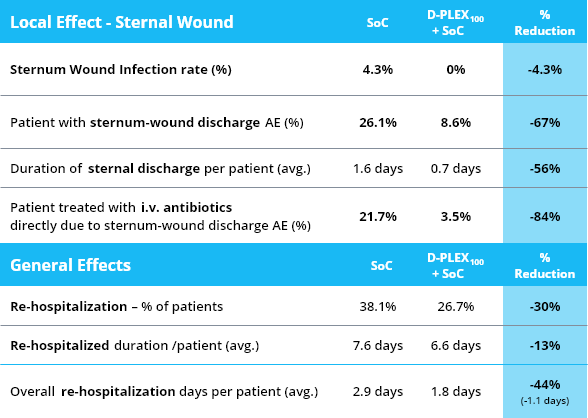
- Primary efficacy endpoint: the decrease of infection rate as measured by the proportion of subjects with at least one sternal infection within 90 days post cardiac surgery
![]()

1 Adding vancomycin to perioperative prophylaxis decreases deep sternal wound infections in high-risk cardiac surgery patients. Reneike S. et al. European Journal of Cardio-Thoracic Surgery (2017) 2 Direct sternal administration of Vancomycin and Gentamicin during closure prevents wound infection. Andreas M. et al. Interactive CardioVascular and Thoracic Surgery (2017) 3Prevention of surgical site sternal infections in cardiac surgery: a two-centre prospective randomized controlled study. Schimmer C et al. European Journal of Cardio-Thoracic Surgery (2016) 4Surgical Site Infections Volume-Outcome Relationship and Year-to-Year Stability of Performance Rankings. Calderwood MS. et al. Med Care 2017;55: 79–85 .
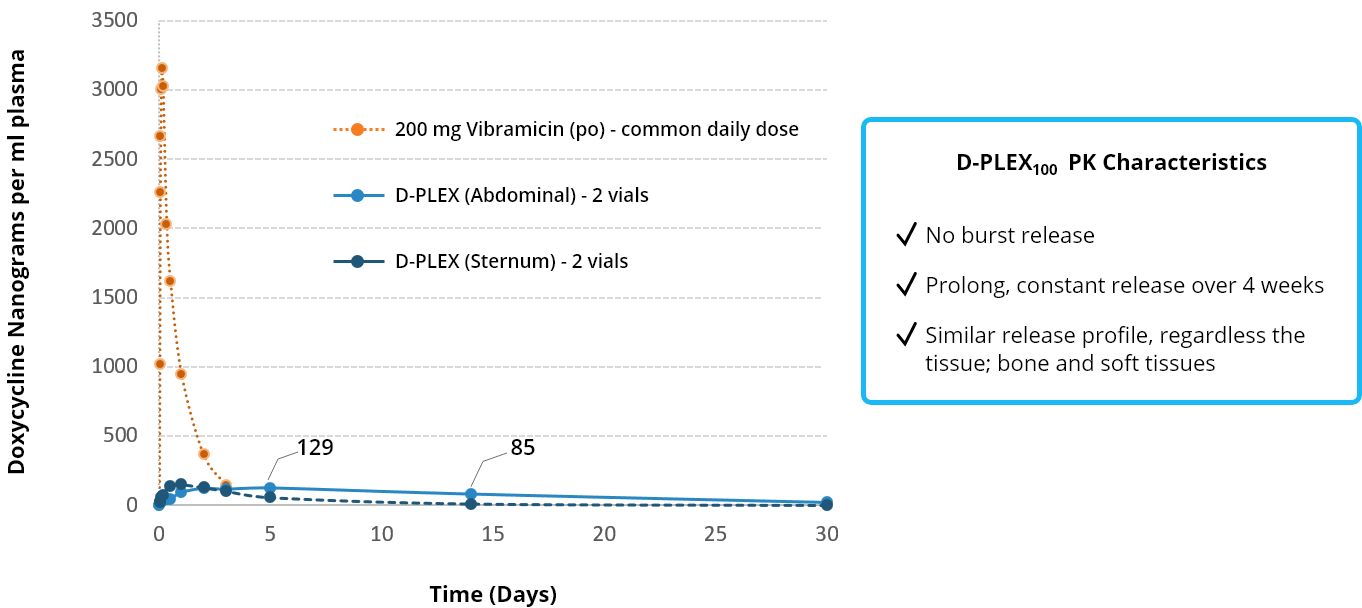
THE LOW SYSTEMIC EXPOSURE OF D-PLEX100 SUPPORTS HIGH SAFETY PROFILE
Comparison D-PLEX100 vs. Systemic administration of Doxycycline –
minimal systemic exposure – Sternum & Abdominal